Zeolite plays a crucial role in gas separation processes. The method of using it as an absorbent is significant because it involves separating the adsorbed substances. The general process includes three main methods:
1. Temperature Swing Adsorption (TSA): This method relies on thermodynamic principles for adsorption and desorption. Adsorption occurs at lower temperatures, while desorption happens at higher temperatures. It uses two reactors: the first is maintained at a low temperature where the target gas is adsorbed. Once the first reactor is saturated, the gas flows into the second reactor for continued adsorption. Meanwhile, the first reactor is heated to desorb the adsorbed substances. When the second reactor is full, the first reactor is ready to resume adsorption. TSA is typically used for adsorbing substances that are stable to heat, such as separating water from natural gas using Zeolite A. This zeolite is highly polar, making it effective at adsorbing water without capturing hydrocarbons. It can also separate H2S from natural gas and SOx and NOx from air.
2. Solvent Replacement: For substances that are unstable to heat and have low vapor pressure, such as hydrocarbons, TSA is not suitable. In these cases, more effective or comparable adsorbents are used. The simplest method is using steam. For example, in adsorbing hydrocarbons with CaA zeolite, steam is more effective at desorbing the hydrocarbons from the zeolite. Afterward, the zeolite is heated to remove the steam from the pores.
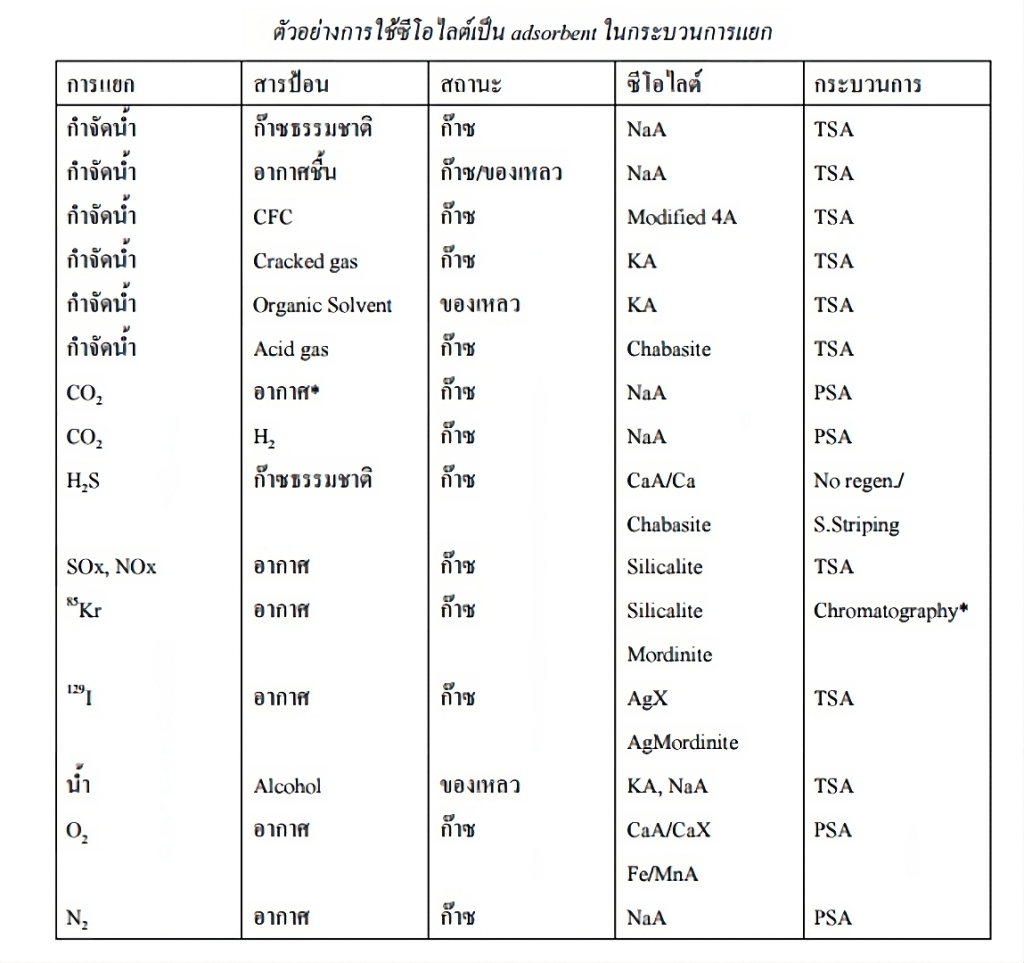
In the petroleum industry, zeolite can be used to separate water from natural gas. Zeolite A, with its high polarity, adsorbs water effectively without adsorbing hydrocarbons, making it suitable for separating natural gas from water.
3. Wastewater Treatment in the Frozen Seafood Industry: Ammonia in wastewater typically appears in the form of ions. Zeolite serves as an excellent medium for adsorption with high cation exchange capacity. It also effectively adsorbs both organic and inorganic substances.



