Zeolite is an oxide compound with acidic properties, featuring a distribution that coexists with silica in a tetrahedral structure. This arrangement causes aluminum atoms in the structure to form ions with a negative charge. As a result, zeolite is balanced by positively charged protons, leading to the formation of strongly acidic zeolite compounds.
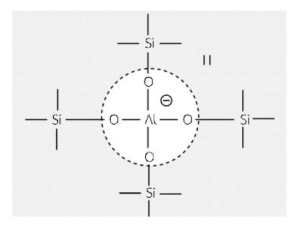
Zeolite compounds exhibit catalytic properties due to their acidic and basic nature:
Acidic Properties: When protons attached to zeolite are released, zeolite exhibits acidic characteristics. For example, if hydrocarbons receive protons released by the zeolite, carbenium ions are formed. These ions play a significant role in the cracking process, which is a method for refining oil.
Petrochemical Processes: Zeolite is used as a catalyst in various petrochemical processes due to its acidic properties, which arise from the protons in its structure. It is employed in processes such as hydrogen cracking, isomer exchange, and alkyl group addition to produce iso-octane, improving combustion performance.
Cracking Process: Zeolite is used as a catalyst in the cracking process, where large hydrocarbons with low utility are broken down into smaller, more valuable hydrocarbons. This process increases the octane rating of fuel and is a method of refining oil. Zeolite and palladium are used as catalysts in this process.
Cracking Reaction Equation
450 องศา
C 10H22 ⇒ C8H16+ C2H6
SiO 2-Al2O3
Removal of Sulfur: Zeolite is utilized in the cracking process to convert paraffins into aromatic compounds, which is an important method for removing sulfur from fuels.
Methanol to Gasoline Process: Zeolite is used in the methanol-to-gasoline process, which converts methanol into gasoline with a higher aromatic content. This process enhances the quality of gasoline by increasing its aromatic hydrocarbon content.



